#"NHF"_2# has #color(red)(7)# lone pairs of electrons, #3# single bonds, and nitrogen has a usual octet. You seem to have counted the three single bonds as additional lone pairs.
#"NHF"_2# is analogous to #"NH"_3#, with two hydrogen atoms replaced by fluorine atoms.
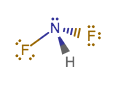
Three lone pairs per fluorine atom gives #6#, plus the lone pair on nitrogen gives #7#. #"N"# atom has the usual octet; two electrons from each single bond and two from the lone pair.

