Question #b5582
1 Answer
(C) (R)-2-chlorobutane and (S)-2-chlorobutane
Explanation:
Potential energy means the energy possessed by a body by virtue of its position relative to others, stresses within itself, electric charge, and other factors and in molecules it could be because of many factors
1.) Strains
2.)dipole moments
3.)bond energies
4.)state of matter and many other....................
Higher potential energy makes a compound less reactive and vice versa.
Specially in organic chemistry no two compounds can be having the same potential energy unless they are enantiomers of each other because their chemical formula, position of atoms,any type of strain and forces are the same in enantiomers.
In these options the only enantiomers are (R)-2-chlorobutane and (S)-2-chlorobutane.
Trans and cis isomers cannot have the same chemical properties

The di-equatorial conformer of trans-1,2-dimethylcyclohexane is the most stable thus it has more potential energy
http://www.chemspider.com/ImagesHandler.ashx?id=4937661&w=250&h=250
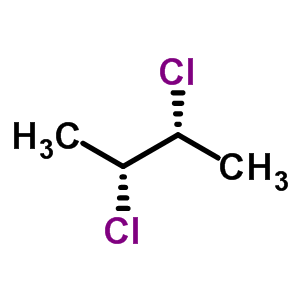
In (2R 3R)-2,3-dichlorobutane there is no prevention of a electrophile by an equatorial atom but in (2R 3S)-2,3-dichlorobutane equatorial atoms are in plenty.This may affect the reactivity
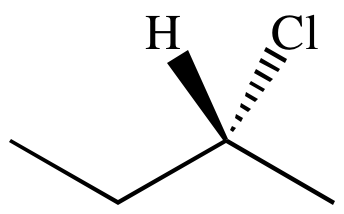
If you switch the places of methyl and ethyl groups
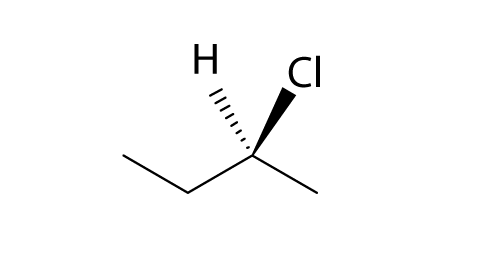
Instead of finding the structures of all the compounds you can reduce your work by not checking the structure of cis and trans isomers
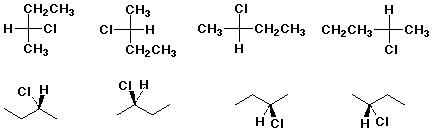
Two projections of the enantiomers
http://structuresearch.merck-chemicals.com/getImage/MDA_CHEM_101692
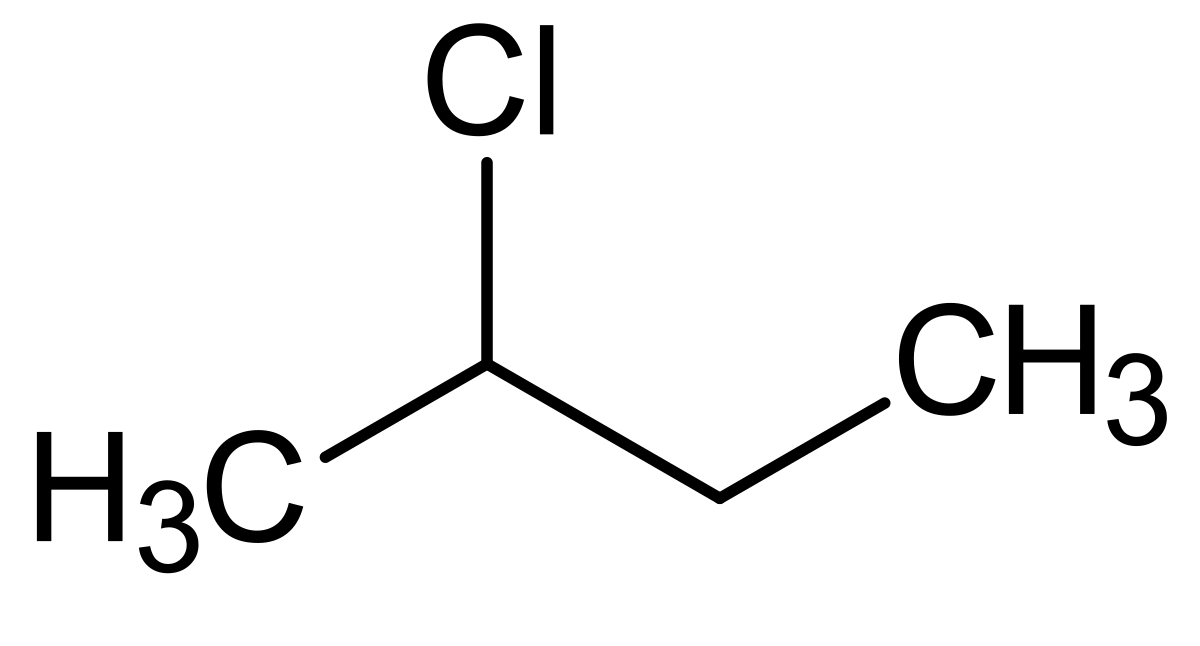
In 1-chlorobutane chlorine is equatorial and in 2-chlorobutane also it is equatorial.But in 1-chlorobutane the carbon in carbon-chlorine bond is tertiary while in 2-chlorobutane it is primary and thus 2-chlorobutane is more reactive.

