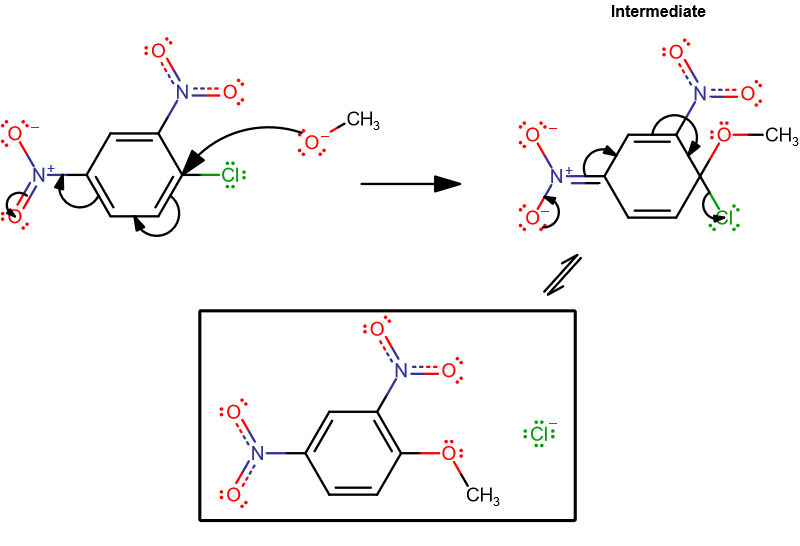
For the nucleophilic aromatic substitution (NAS) reaction of 1-chloro-2,4-dinitrobenzene with methoxide in methanol, what does the intermediate look like?
2 Answers
option (B)
Explanation:
First keep in mind that methanol is not donating the
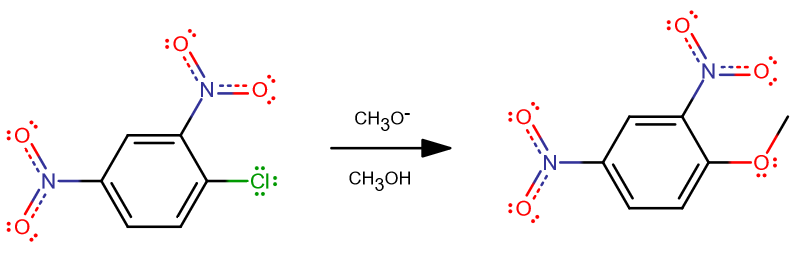
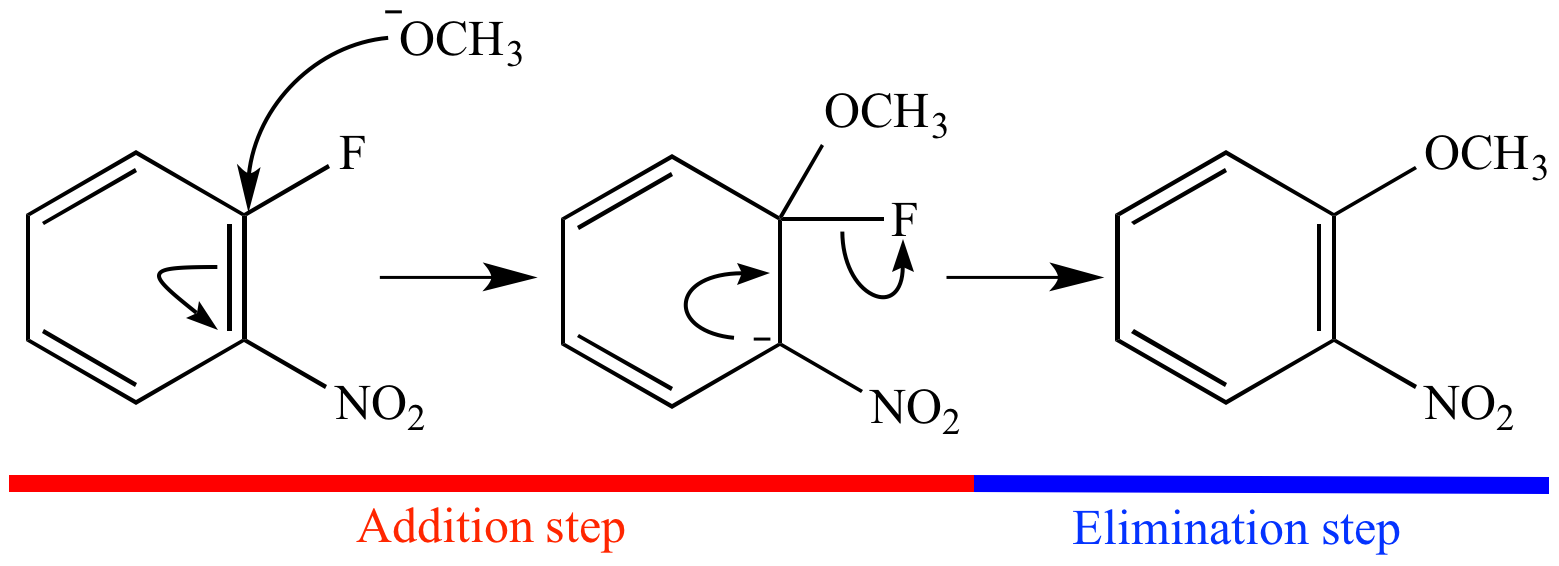
This reaction is Nucleophilic aromatic substitution and a SNAr reaction. The 'Ar' stands for 'aromatic'.
First step) a very strong nucleophile (
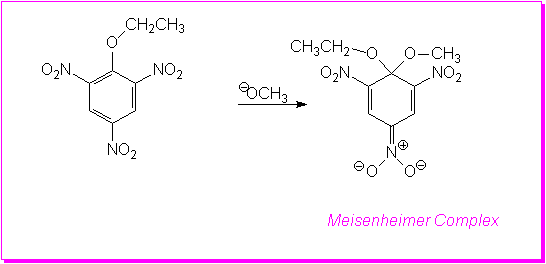
This photo is just an example.Not to be thought as the orginal reaction
2nd step) The reaction proceeds forward via the elimination of chlorine.Please keep in mind that the (
The intermediate is shown below in the diagram at the bottom.
The reaction you have is:

The nitro (
Having two of them on the same benzene ring promotes nucleophilic aromatic substitution, where a nucleophile can displace a ligand.
Here, the nucleophile is