Given combustion data, how would we find the empirical formula of #C_7H_5N_3O_6#?
1 Answer
Explanation:
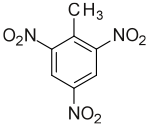 http://en.wikipedia.org/wiki/TNT
http://en.wikipedia.org/wiki/TNT
The
And you were given the elemental composition that IGNORED the oxygen atoms, and got the number of hydrogen atoms wrong.
We gots
TNT is used as an explosive because of its ease of manufacture, and its resistance to detonation under standard conditions. Of course, when properly primed, and detonated, it is a highly potent explosive.
Note that when microanalysis is performed upon an organic sample, we measure

