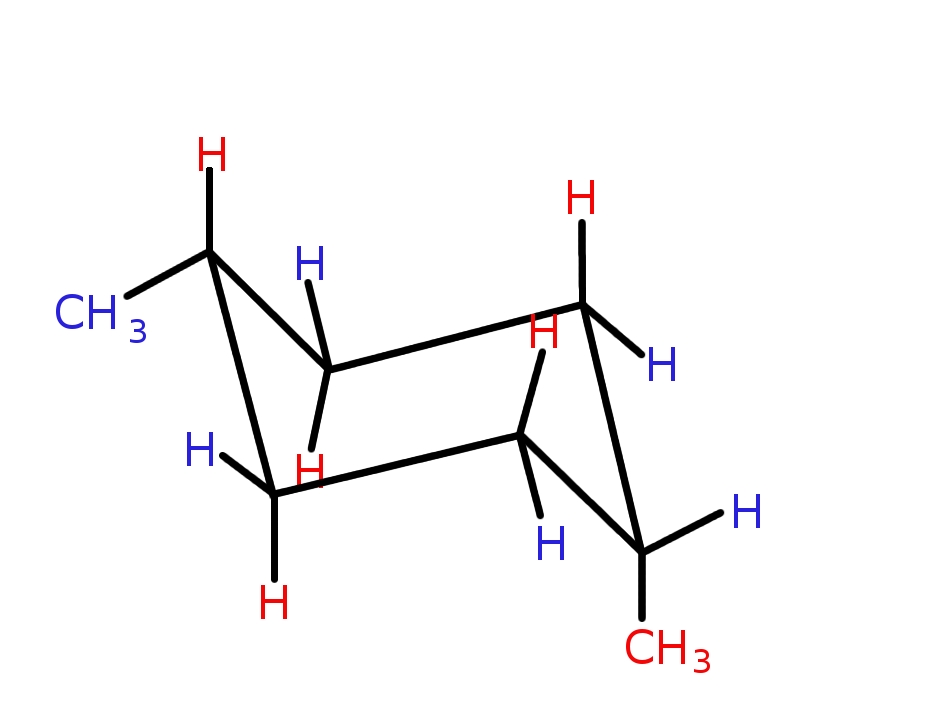
The given molecule is #"cis-1,4-dimethylcyclohexane"#, where one of the methyl substituents is AXIAL, and the other is EQUATORIAL. The methyl groups can exchange their orientation with respect to the ring, by flipping the ring, and there is a fairly soft energy of exchange. A different molecule is #"trans-1,4-dimethylcyclohexane"#, where the methyl groups can be trans-diaxial or trans-diequatorial.
For your problem, you have (i) got to make a model that represents #"methylcyclohexane"#, and (ii) represent that model on the printed page. Neither is facile, and it does take practice to draw a 3D structure on the printed page - don't leave it until the exams to practise.


