Given the quoted data, which alcohol is the LEAST stable of the three butyl alcohols?
#bb(color(white)(mmmmmmm)"Heat of Combustion" #
#bbul(color(white)(mmmm)"1-Butanol"color(white)(m)"2-Butanol"color(white)(m)"Isobutyl alc." #
#"Test 1"color(white)(mml)3236.20color(white)(mmm)3248.66color(white)(mmml)2663.82 #
#"Test 2"color(white)(mml)3833.22color(white)(mmm)2846.88color(white)(mmml)2915.03 #
#ul("Test 3"color(white)(mml)2823.51color(white)(mmm)2861.16color(white)(mmml)2992.80)#
#"Averages"color(white)(m)3300color(white)(mmmml)2990color(white)(mmmmm)2860#
2 Answers
Your data are very hard to interpret..........and if you want a complete answer you need to supply a complete question...you ain't even got units quoted.
Explanation:
I suspect that there is no difference in the enthalpies you measured; i.e. the variation in values stems from random error.
For the combustion of butanol, isobutanol, and tert-butanol, we gots....
I suspect that given complete combustion there would be very little difference in the absolute enthalpies. The branched alcohols MIGHT combust LESS efficiently, giving rise to reduced heat output, with respect to
Butan-1-ol is the least stable (highest energy) compound of the three alcohols.
Explanation:
Here is what I think your data are:
Now, let's display them on an energy level diagram:
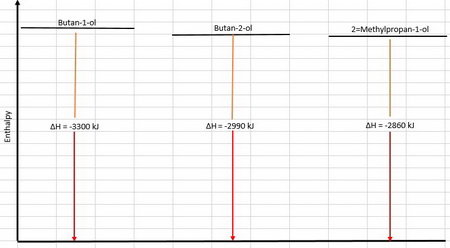
Relative stabilities
All the compounds end up at the same energy level (as
The less stable compound is at a higher energy level and therefore releases the most energy on combustion.
Thus, the order of stabilities is
Why this order?
It appears to be related to the amount of branching in the alcohols.
If we count the
Theoretical calculations suggest that the order is caused by overlap between a
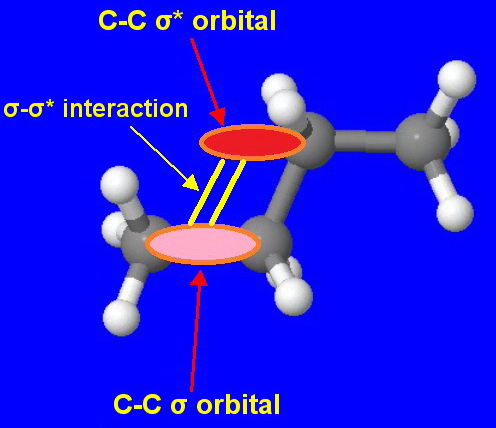
The effect is small but, the more highly branched the molecule, the more of these interactions there will be, and the compound will be more stable.


