How many signals should be observed in the #""^1H# #"NMR spectrum"# of #"methyl benzoate?"#
1 Answer
Well, you gots
Explanation:
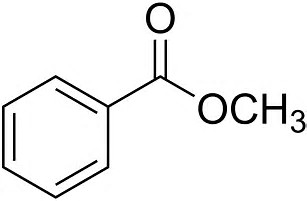
I think you should see 4 peaks in a
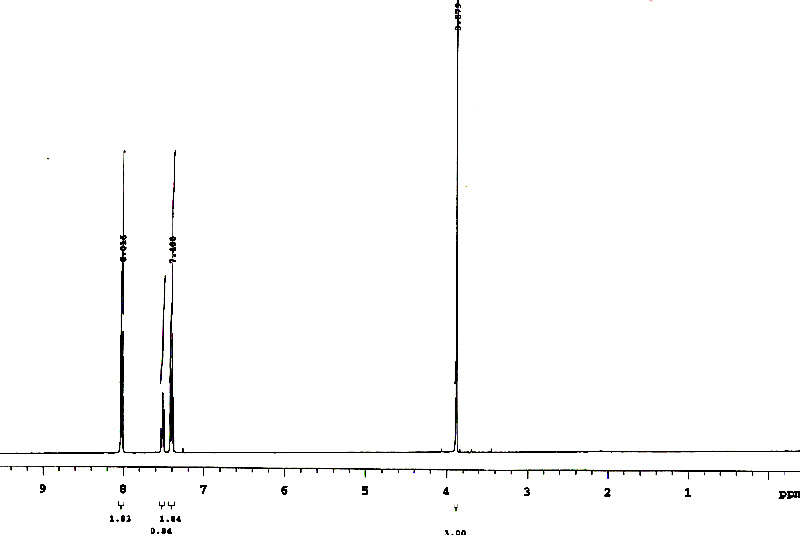
And we see the four required peaks, and with (almost) the required integration ratio.....
SO how did I know that methyl benzoate would yield 4 peaks?
Well, this is where we use symmetry to examine the molecule. Any group of set of protons that can be interchanged by symmetry or a fast moving process, are said to be
If you are not sure which is which, we can use integration to refine our assignment. The methyl hydrogens, and the para hydrogen are unequivocally identified by their integration ratio. We don't know which of the remaining signals belongs to ortho or meta, but this really does not matter.
By contrast, the
And luckily, we have got....
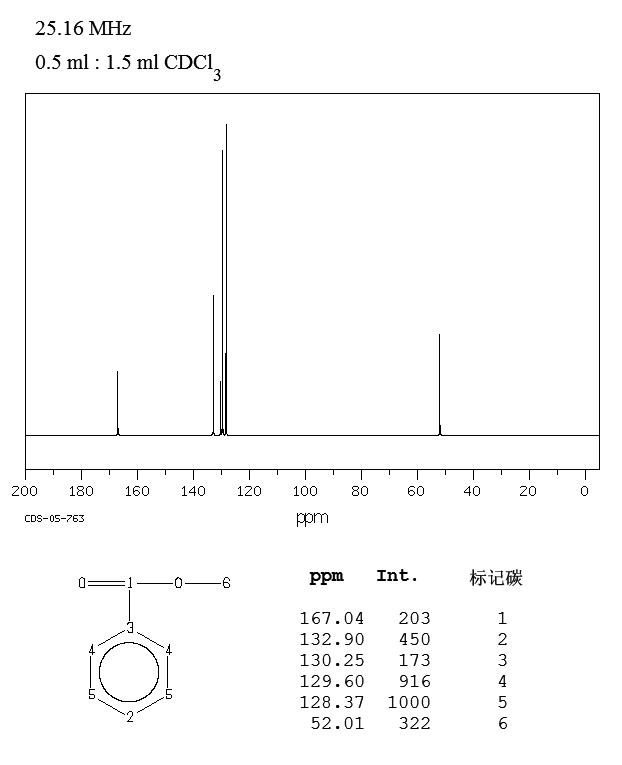
And thus there are 6 carbon signals as required. It should be fairly easy to differentiate the aryl signals from the carbonyl and the methyl signals............

