What is the strongest intermolecular force? Identify the strongest intermolecular forces in methanol and carbon tetrachloride. How do your answers explain what happens when you add methanol to carbon tetrachloride?
2 Answers
Well intermolecular hydrogen bonding operates in methanol.....
Explanation:
And only dispersion forces operate in carbon tetrachloride. We might assume that the latter is more volatile than methanol. But as physical scientists, we should consider the data, and the best metric of intermolecular attraction should be normal boiling point...
So in fact carbon tet is LESS volatile than methanol. Mind you it is a much heavier molecule than methanol, with more electrons, and thus greater opportunity for dispersion forces. A better molecule for comparison might be chloromethane, or methylene chloride....
Are you happy with this rationale?
PS I do not even know whether methanol is soluble in carbon tet. Is it? This is the province of experiment. Certainly, methanol is INSOLUBLE in hexane,
The strongest intermolecular force is a dipole-induced dipole interaction.
Explanation:
Intermolecular forces
The three most common intermolecular forces, from strongest to weakest are:
• dipole - dipole (which includes hydrogen bonds)
• dipole - induced dipole
• London dispersion forces
Intermolecular forces in methanol
The strongest intermolecular forces in methanol are hydrogen bonds ( an especially strong type of dipole-dipole interaction).
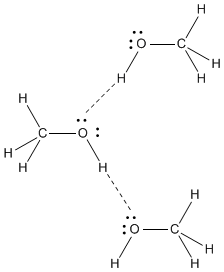
Intermolecular forces in
The
Thus,
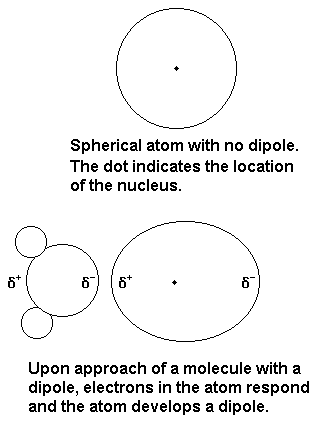
We tend to think of
However, the electron cloud around the molecule is nearly spherical, as in the diagram above.
The major interaction between