What is the structure of #"hexane"#? How is this structure reflected in its #""^1H# #"NMR spectrum"#?
1 Answer
Well, the molecule is linear to a first approx.....
Explanation:
But in isotropic solution, the molecule is conceived to tumble about. Spectroscopic techniques suggest that can interrogate the molecular symmetry, i.e.
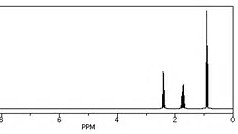
........and the
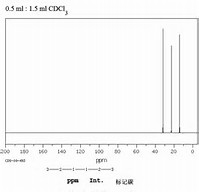
Both spectra give the required three signals for the
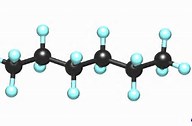
And as a general rule, when given an organic molecule to consider, it is usually a good idea to represent it on paper as symmetrically as possible. If something is funny with its preferred conformation, you will soon find out.

