Question #d4b1b
1 Answer
Here's what I get.
Explanation:
Step 1. Formation of the phenylhydrazone
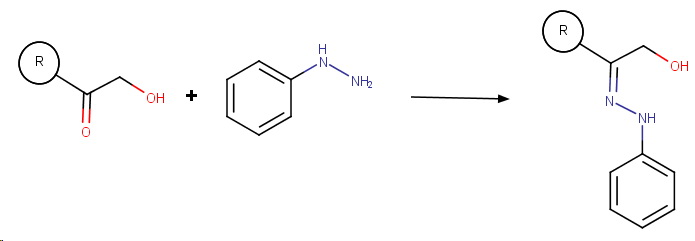
Here, I show only carbons 1 and 2 of fructose.
The mechanism is that for the base-catalyzed condensation of an amine with a ketone.
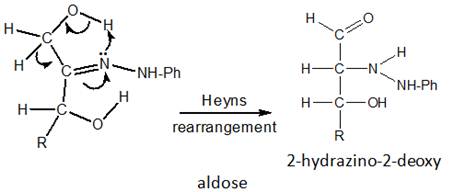
Step 2. Thermal Rearrangement
The phenylhydrazone undergoes a thermal rearrangement (Heyns rearrangement) via a cyclic concerted five-member ring.
Step 3. Formation of the aldehyde phenylhydrazone
The aldehyde group reacts with more phenylhydrazone ( the first step in the reaction scheme below).
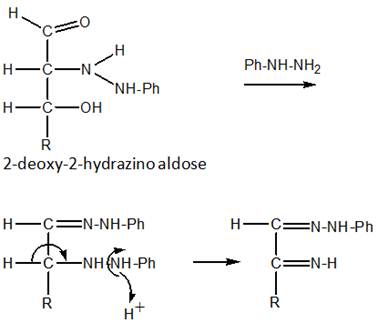
Step 4. Elimination of aniline
The product then eliminates a molecule of aniline to form an α-ininophenylhydrazone (the second step in the scheme above).
Step 5. Formation of the osazone
Finally, the imine group condenses with another molecule of phenylhydrazine to form the osazone.
The general equation is

