How many isomers can a formula of C_4H_10O generate?
2 Answers
Well, I count 7 isomers.....
Explanation:
There are four alcohols....
And three ethers.....
You should draw the structures yourself. There is no better training for recognizing, and numbering isomers.
Here's what I get.
Explanation:
Structural isomers
Structural Isomers are molecules with the same molecular formula but different connectivities (the atoms are connected in different orders).
For example, two structural isomers of
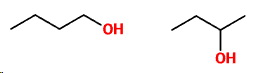
The
Two other structural isomers of

Again, the
Functional group isomers
Functional group isomers are molecules with the same molecular formula but different functional groups.
For example, butan-1-ol and diethyl ether are functional group isomers.

They have the same molecular formula, but one is an alcohol and the other is an ether.


