Question #7cb5a
1 Answer
yes, in a way....
or no, depends how you look at it.
Explanation:
A Glycosidic bond is a (covalent) bond between two carbohydrates e.g. Fructose and Glucose to form Sucrose:
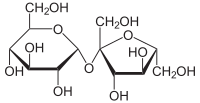
or between a carbohybrate and another molecule, like in glycosylation of proteins.
An ester bond, on the other hand, is a bond between an acid and an other molecule, usually an alcohol, whereby at least one OH-group is replaced by an OR-group, in which the R usually is an alkyl group:
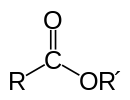
It usually concerns organic acids like formic, acetic or propionic acid etc., but inorganic esters (made with an inorganic acid) also exist.
And yes, (and this is probably why the question was asked in the first place) the backbones of both DNA and RNA ARE formed by phosphodiester bonds, bonds between a phosphate molecule and.......
2 Sugars! (2 molecules of 2-D-deoxyribose in DNA or D-ribose in RNA):
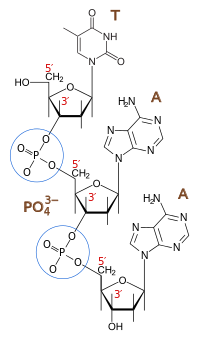
But that doesn't mean they are the same, because in the formation of EthylAcetate for instance, not a sugar in sight.....
As a foot note: interestingly enough, you encounter Esters every day of your life: they are very fragrant, and are the chemicals that give fruits, vegetables etc. their smell (Ethyl Acetate smells like peardrops, and that is probably what they put in them anyway....)

