Question #a12ff
1 Answer
Explanation:
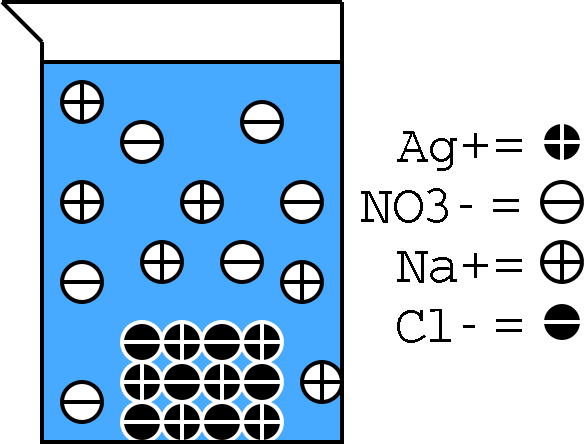
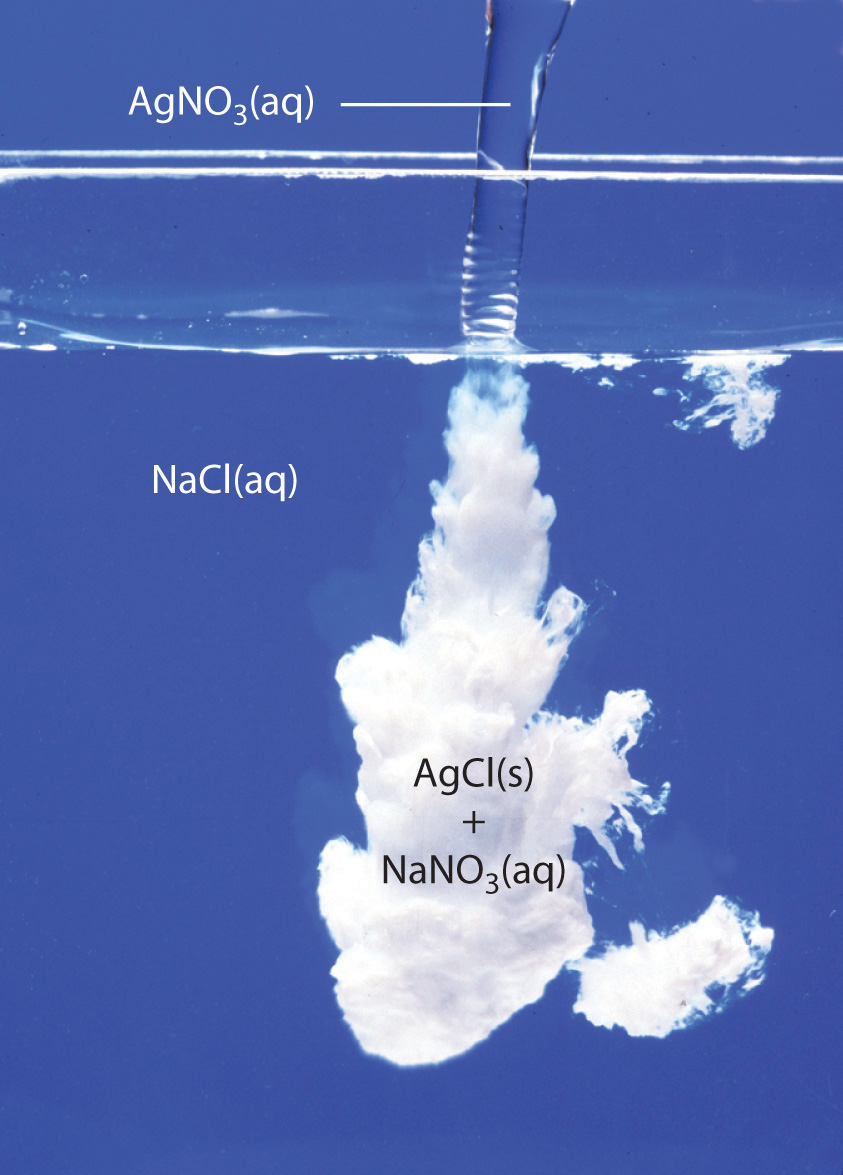
Silver nitrate and sodium chloride will react in aqueous solution to produce silver chloride, an insoluble solid that precipitates out of the solution, and aqueous sodium nitrate.
The balanced chemical equation that describes this double-replacement reaction looks like this
#"AgNO"_ (3(aq)) + "NaCl"_ ((aq)) -> "AgCl"_ ((s)) darr + "NaNO"_ (3(aq))#
Silver chloride is a white insoluble solid that will precipitate out of the solution.
If you want, you can use the fact that silver nitrate and sodium chloride are soluble in water--the same can be said about sodium nitrate, the second product of the reaction--to write the complete ionic equation that describes this reaction.
#overbrace("Ag"_ ((aq))^(+) + "NO"_ (3(aq))^(-))^(color(blue)("AgNO"_ (3(aq)))) + overbrace("Na"_ ((aq))^(+) + "Cl"_ ((aq))^(-))^(color(blue)("NaCl"_ ((aq)))) -> "AgCl"_ ((s)) + overbrace("Na"_ ((aq))^(+) + "NO"_ (3(aq))^(-))^(color(blue)("NaNO"_ (3(aq))))#
If you eliminate the spectator ions, which are the ions present on both sides of the equation
#"Ag"_ ((aq))^(+) + color(red)(cancel(color(black)("NO"_ (3(aq))^(-)))) + color(red)(cancel(color(black)("Na"_ ((aq))^(+)))) + "Cl"_ ((aq))^(-) -> "AgCl"_ ((s)) darr + color(red)(cancel(color(black)("Na"_ ((aq))^(+)))) + color(red)(cancel(color(black)("NO"_ (3(aq))^(-))))#
you will get the complete ionic equation
#"Ag"_ ((aq))^(+) + "Cl"_ ((aq))^(-) -> "AgCl"_ ((s)) darr#