Question #417d7
1 Answer
Jan 5, 2018
Here's how I would do it.
Explanation:
It consists of
The
Then you draw the Lewis structure of the
You have
This gives a Lewis structure of
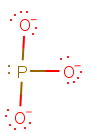
The structure has four electron domains, so its electron geometry is tetrahedral and its molecular geometry is trigonal pyramidal.