Question #aa7ee
1 Answer
Dec 30, 2017
An ionic bond.
Explanation:
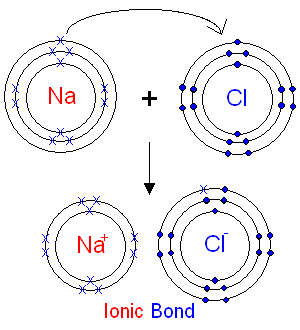
An ionic compound consists of oppositely-charged ions held together by electrostatic forces called ionic bonds.
The ions are formed by the transfer of electrons between atoms.
For example, we can think of the formation of
 www.gcsescience.com
www.gcsescience.com
The
The
The electrostatic force of attraction between the oppositely charged ions is called an ionic bond.

