Question #5dc82
1 Answer
Here's what I got.
Explanation:
The first thing that you need to do here is to calculate the wavelength of the photon emitted when your electron undergoes a
The wavelength of the photon will then help you determine its energy.
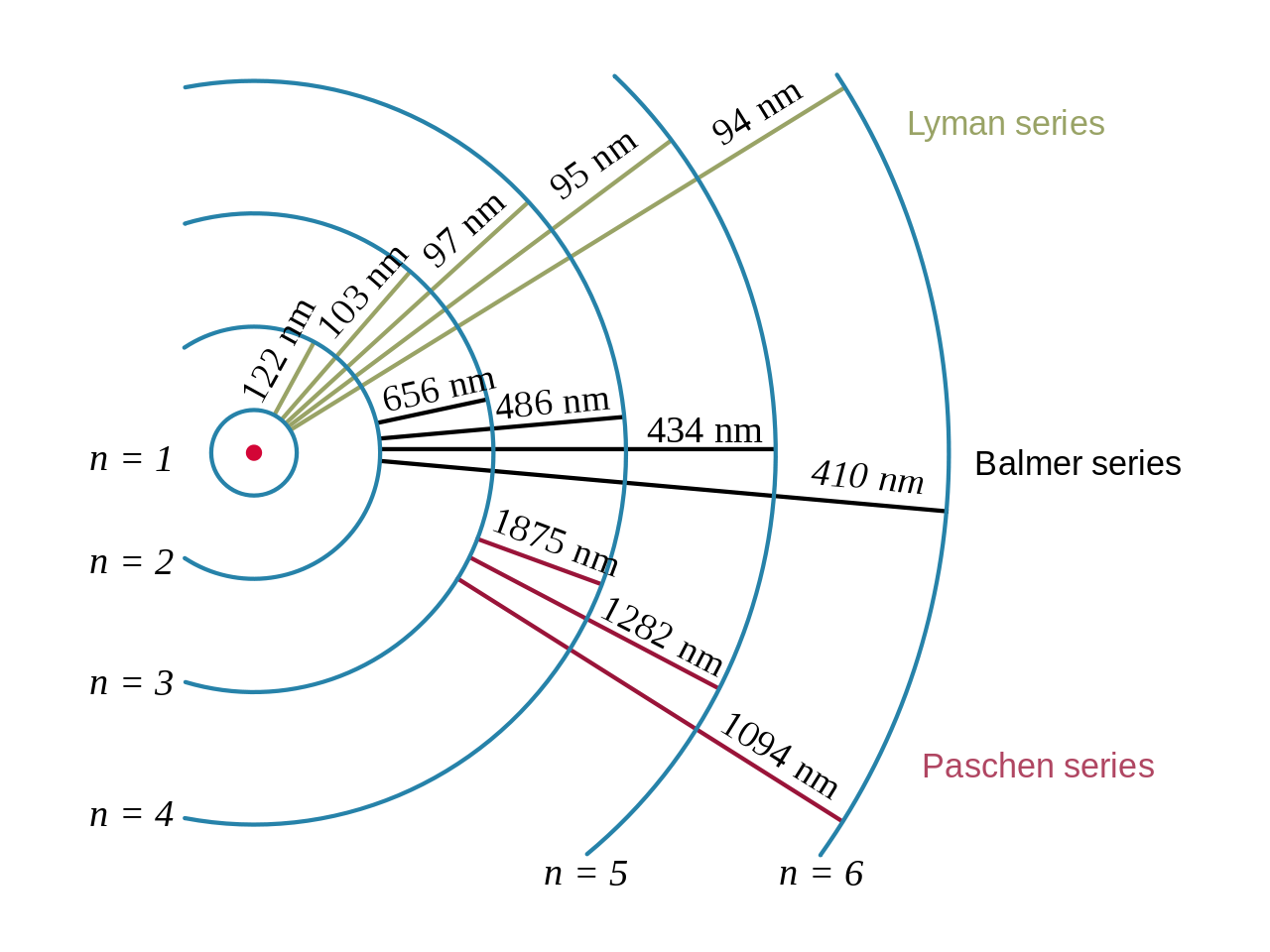
So, you know that when an electron in a hydrogen atom makes a transition from an initial energy level
#1/(lamda) = R * (1/n_f^2 - 1/n_i^2)#
Here
#R# is the Rydberg constant, equal to#1.097 * 10^(7)# #"m"^(-1)#
You can rearrange the above equation, which is called the Rydberg equation, as
#1/(lamda) = R * (n_i^2 - n_f^2)/(n_i^2 * n_f^2)#
which gets you
#lamda = 1/R * (n_i^2 * n_f^2)/(n_i^2 - n_f^2)#
In your case, you have
#lamda = 1/(1.097 * 10^7color(white)(.)"m"^(-1)) * (5^2 * 2^2)/(5^2 - 2^2)#
#lamda = 4.34 * 10^(-7)color(white)(.)"m"#
Expressed in nanometers--recall that
#lamda = color(darkgreen)(ul(color(black)("434 nm")))#
Now, in order to find the energy of the photon, you need to use a variation of the Planck - Einstein relation.
#E = h * c/(lamda)#
Here
#E# is the energy of the photon#h# is Planck's constant, equal to#6.626 * 10^(-34)# #"J s"# #c# is the speed of light in a vacuum, usually given as#3 * 10^8# #"m s"^(-1)#
Plug in your value to find
#E = 6.626 * 10^(-34)"J" color(red)(cancel(color(black)("s"))) * (3 * 10^8 color(red)(cancel(color(black)("m"))) color(red)(cancel(color(black)("s"^(-1)))))/(4.34 * 10^(-7) color(red)(cancel(color(black)("m"))))#
#E = color(darkgreen)(ul(color(black)(4.58 * 10^(-19)color(white)(.)"J")))#
I'll leave both answers rounded to three sig figs.
It's worth mentioning that the