Question #61c84
1 Answer
Oct 11, 2017
Gold will have the highest final temperature.
Explanation:
This is tricky because one's intuition sees
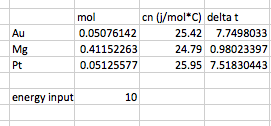
In English, this table means that if I input

