How does atomic size evolve with respect the position of an element in the Periodic Table?
1 Answer
It is a fact that atomic size decreases across a Period....
Explanation:
It is a fact that atomic size DECREASES across a Period, a row of the Periodic Table, from left to right as we face the Table, and INCREASES down a Group. And this is something I would expect a novice student of chemistry to grasp in that it reflects the structure of the Periodic Table.
Incomplete electronic shells shield the nuclear charge very imperfectly, and as we add protons to the nuclear core (thereby changing the identity of the element) the added electrons shield the nuclear charge VERY imperfectly....
And this results in a contraction of atomic radius across the Period..
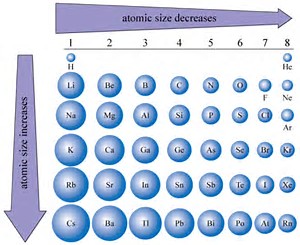
But when a valence shell is completed (i.e. at the Noble gases, which are the smallest atoms of their Periods), the shielding of nuclear charge becomes effective. The next electron adds to a new valence shell. And the macroscopic observable is the LARGE size of the alkali metal atoms in relation to the succeeding atoms in their Periods.
And so for sulfur, sodium, and chlorine ATOMS, we would predict that atomic size decreases in that order.....We would also predict that sodium atom is LARGER than lithium atom, but we do not really have a handle when we compare the atomic size of lithium versus that of sulfur, and chlorine... From the graph, lithium is larger than sulfur, and chlorine, even tho' lithium is a second Period atom.

