Why are #pi# orbitals doubly-degenerate?
1 Answer
Oct 15, 2017
Because they formed from the equivalent sidelong interactions of
There is no difference, in principle, between the
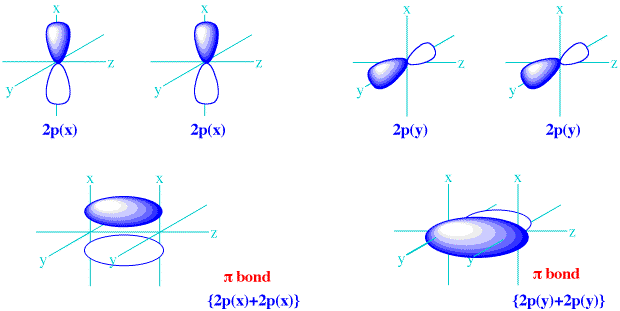
In fact, if you envision using your right hand to take the
So, they are the same energy molecular orbitals.

