What happens if an atom is not neutral?
1 Answer
Oct 22, 2017
It will become either a cation, or an anion.
Explanation:
If an atom is not neutral, then it will form an ion. In this case, the number of protons and electrons are not equal.
If the atom loses one or more electrons, it will have more protons than electrons, and will form a positively charged ion (cation).
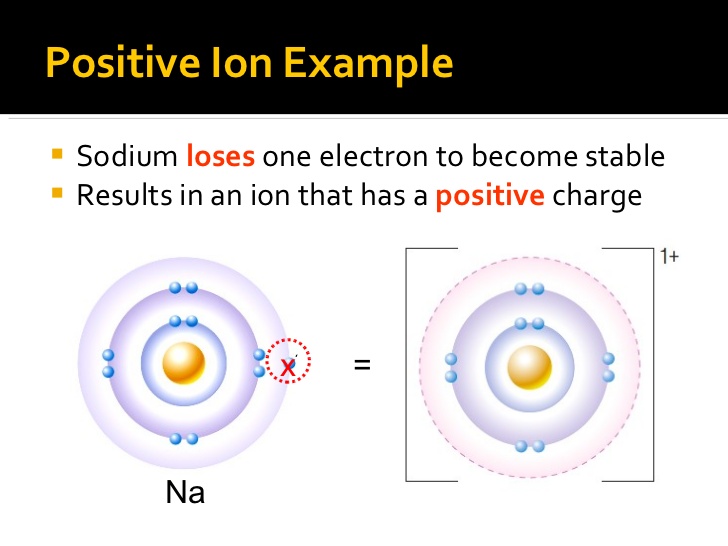 https://www.slideshare.net/thelawofscience/ions-ionic-compounds
https://www.slideshare.net/thelawofscience/ions-ionic-compounds
If the atom gains one or more electrons, it will have more electrons than protons, and will form a negatively charged ion (anion).
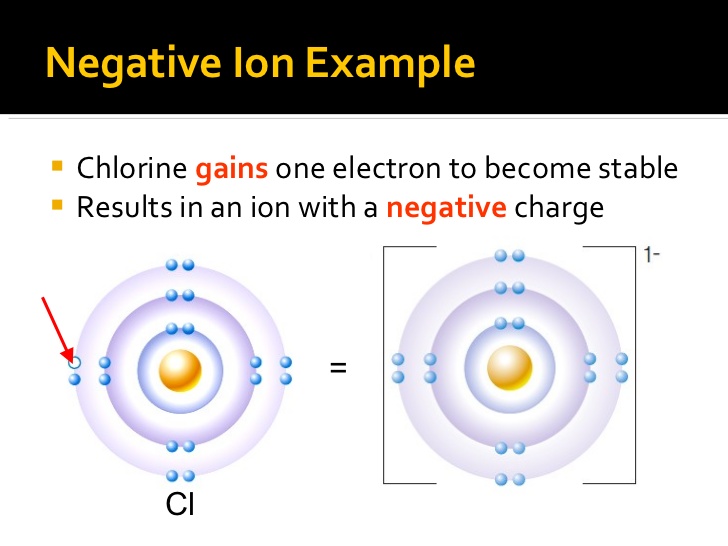 https://www.slideshare.net/thelawofscience/ions-ionic-compounds
https://www.slideshare.net/thelawofscience/ions-ionic-compounds

