How is energy plotted against the reaction coordinate in an exothermic reaction?
1 Answer
Oct 17, 2017
Surely there is such a graph in your text....?
Explanation:
For an exothermic reaction, the enthalpy of the products is LESS than the enthalpy of the reactants, and heat is lost to the surroundings. TO represent this graphically.....
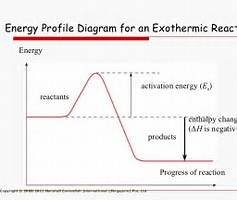
Energy/enthalpy is plotted against the reaction coordinate, i.e. the progress of reaction as reactants are transformed into products. There is usually an associated activation energy, an energy barrier that the reactants must overcome for successful reaction. The use of catalysis can affect the activation energy, but has no effect on the enthalpy change of an exothermic reaction

