Question #b4ab9
1 Answer
Here's what I got.
Explanation:
For starters, calculate the charge of the ion by using the equation
#color(blue)(ul(color(black)("net charge" = "no. of protons " - " no. of electrons"#
In your case, you have
#"net charge" = 52 - 54 = -2#
This tells you that the ion carries a
Now, the isotope notation of this ion requires
- the atomic number of the element,
#Z# - the mass number of the isotope,
#A#
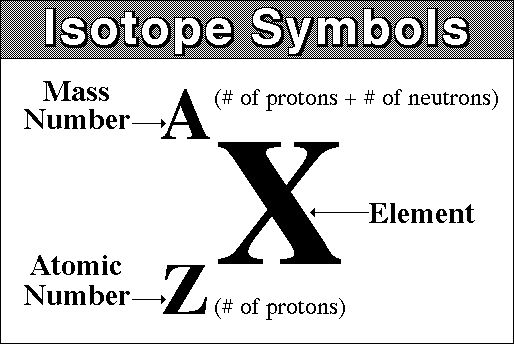
In your case, you know that the element has
#Z = 52#
In order to find the mass number of the isotope, simply add the number of protons and the number of neutrons present in the nucleus.
#A = "52 protons + 76 neutrons"#
#A = "128 nucleuons"#
This means that the symbol of the neutral isotope will look like this
#""_(color(white)(1)52)^128"X"#
A quick look in the Periodic Table will reveal that you're dealing with tellurium-128, an isotope of tellurium,
#""_(color(white)(1)52)^128"Te"#
Finally, to show that this is an anion and not a neutral atom, add the net charge.
#""_(color(white)(1)52)^128"Te"^(2-)#

