What is the wavelength for light of frequency #1xx10^-13*s#?
1 Answer
Oct 25, 2017
WE use the relationship
Explanation:
And thus
And if
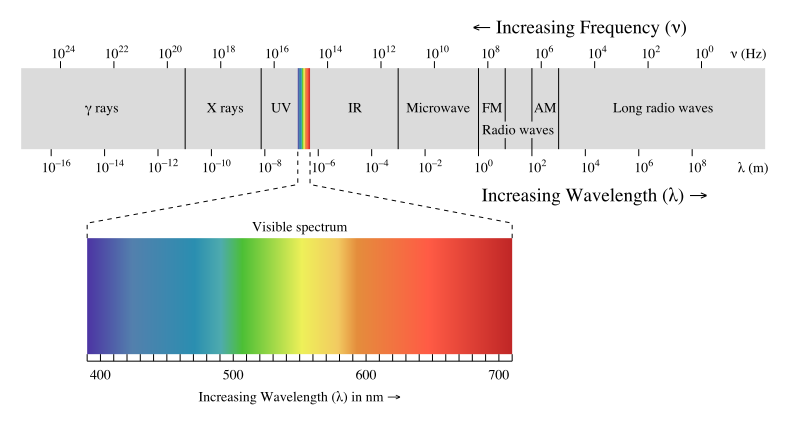
...and this is in the radiowave region.....
I leave it to you to calculate the other wavelengths....

