Question #e91e6
1 Answer
Oct 30, 2017
An addition reaction usually occurs when the valency of the reacting substance is not satisfied.
While the need of substitution reaction arises when we want to change completely the nature of a chemical compound. Because in these reactions functional groups in chemical compound is replaced.
Explanation:
Lets check out some simple examples...
Example for Addition reactions
- Hydrogenation of alkene and alkynes:
As in alkenes(having double bond) and akynes(having triple bond), the valency of carbon atom is not fully satisfied because of the presence of double bond. That's why hydrogen is added to them to have saturated compounds i.e alkanes having sinsle bonds between them and thus fully satisfied valency of carbon atom.
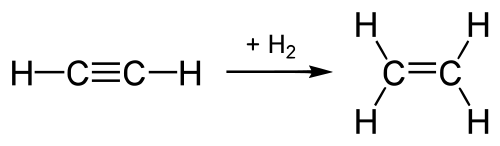
[https://commons.wikimedia.org/wiki/File:Synthesis_Ethylenesvg]
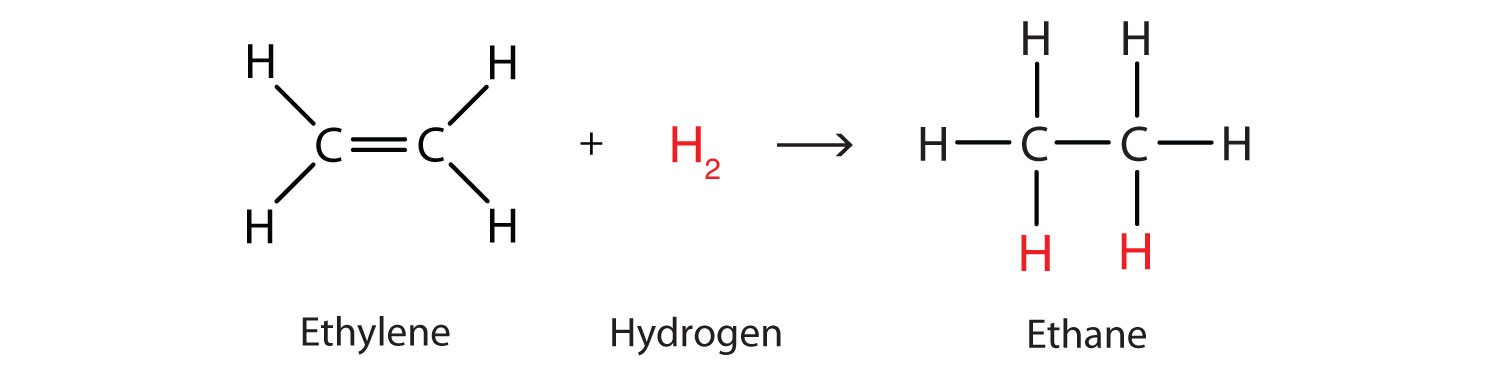
(http://lucychemistry.blogspot.com/2013/05/38-describe-addition-reaction-of.html)
Example for Substitution Reactions
#SN_2# reactions of Alkyl halides:
Nucleophilic bimoecular reactions are called#SN_2# reactions.
In these reactions the halogen group of alkyl halide molecule is replaced or substituted by the attacking nucleophile and changes the alkyl halide to an alcohol.
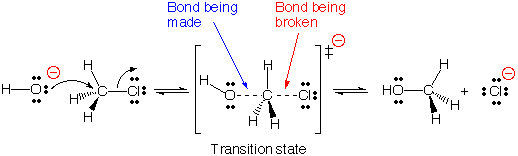
[(http://iverson.cm.utexas.edu/courses/310N/ReactMoviesFl05%20/SN2text.html) ]

