Question #c5c5d
1 Answer
Nov 12, 2017
The tricyclopropylmethyl cation is more stable than the triphenylmethyl cation.
Explanation:
Triphenylmethyl cation
The triphenylmethyl cation is relatively stable because of resonance stabilization of the aromatic rings.

However, the resonance is not perfect because steric hindrance of the ortho hydrogens causes the ion (and the radical) to assume a propeller shape.

Tricyclopropylmethyl cation
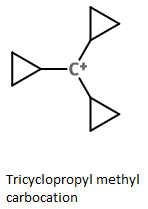
Cyclopropyl groups are much more effective than phenyl groups in stabilizing a positive charge.
The bent bonds of the cyclopropane ring can overlap with the empty
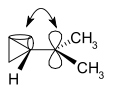
(Adapted from Wikipedia)

