Why is each ion in the sodium chloride lattice six-coordinate?
2 Answers
You mean with respect to the solid-state structure....?
Explanation:
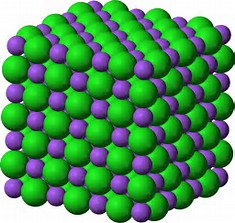
We could describe the structure as an infinite array of close-packed sodium cations, with chloride anions in all the octahedral holes, or vice versa as an infinite array of close-packed chloride anions, with sodium cations in all the octahedral holes...Each sodium ion has 6 chloride nearest neighbours, and each chloride ion has 6 sodium nearest neighbours....because this is the MOST stable structure energetically...
Of course, the sodium ions repel each other electrostatically, as do all the halide ions, but if you sum up electrostatic attraction between unlike charges, versus electrostatic repulsion between like charges (and this can certainly be done quantitatively), attraction wins, and there is net force of electrostatic attraction that binds the lattice together.... And this is the stable arrangement energetically.....
Each ion in the sodium chloride lattice is six-coordinate because it has six nearest neighbours.
Explanation:
The sodium chloride crystal structure consists of a face-centred cubic lattice.
(Adapted from Chemguide)
A sodium ion sits at the centre of the cube, and its nearest neighbours are the chloride ions at the centre of each of the six faces: left-right, top-bottom, front-back.
We can also draw the unit cell with a chloride ion at the centre of a face-centred cube.
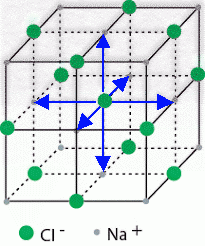
(Adapted from nptel.ac.in)
The nearest neighbours of the chloride ion are the sodium atoms at the centre of each of the six faces: left-right, top-bottom, front-back.
Thus, both the sodium ions and the chloride ions are six-coordinate.


