MO diagram for #"C"_2"^(2-)#?
1 Answer
Nov 15, 2017
Here's what I get.
Explanation:
We want to derive the MO diagram for
Each
Below is an MO energy level diagram for the diatomic entities of
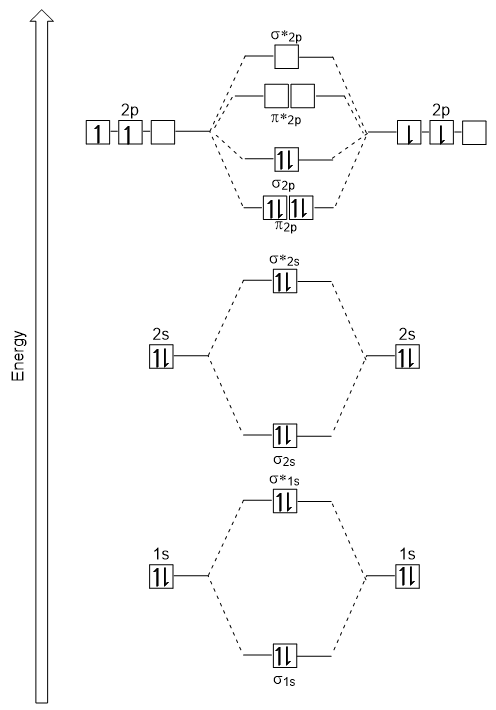
(Adapted from BC Open Textbooks)
We fill the boxes following the Aufbau Principle and Hund's Rule until we have added 14 electrons.
Thus, the sequence of molecular orbitals in

