How many right angles are there in a XeF+5 ion?
1 Answer
Nov 16, 2017
There are eight right angles in
Explanation:
You must draw the Lewis structure of
Five
Your Lewis structure will have 5
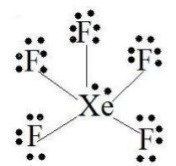 XeF5+
XeF5+
(Adapted from SlideShare)
This is an
The electron geometry is octahedral
 dublinsciotochemistry.wikispaces.com
dublinsciotochemistry.wikispaces.com
The molecular geometry is square pyramidal.
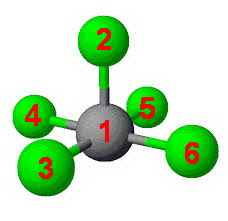 Square pyramidal
Square pyramidal
(Adapted from Ask the TA)
Now, we count the right angles.
Using the numbers of the atoms, we get the angles:
- 2-1-3
- 2-1-4
- 2-1-5
- 2-1-6
- 3-1-4
- 3-1-6
- 4-1-5
- 5-1-6
That makes eight right angles.

