Question #90336
1 Answer
Elements in a group have the same number of outer shell electrons but occupy additional energy levels.
Explanation:
The group (family) of an element on the periodic table denotes elements with similar chemical properties. For example, all of the Group 1 alkali metals - lithium, sodium, potassium for example - have similar reactions to water. Since the way an element reacts is affected by its electronic configuration, it makes sense to reason that elements in a group react similarly because of similar electronic configurations.
Take the alkali metals.
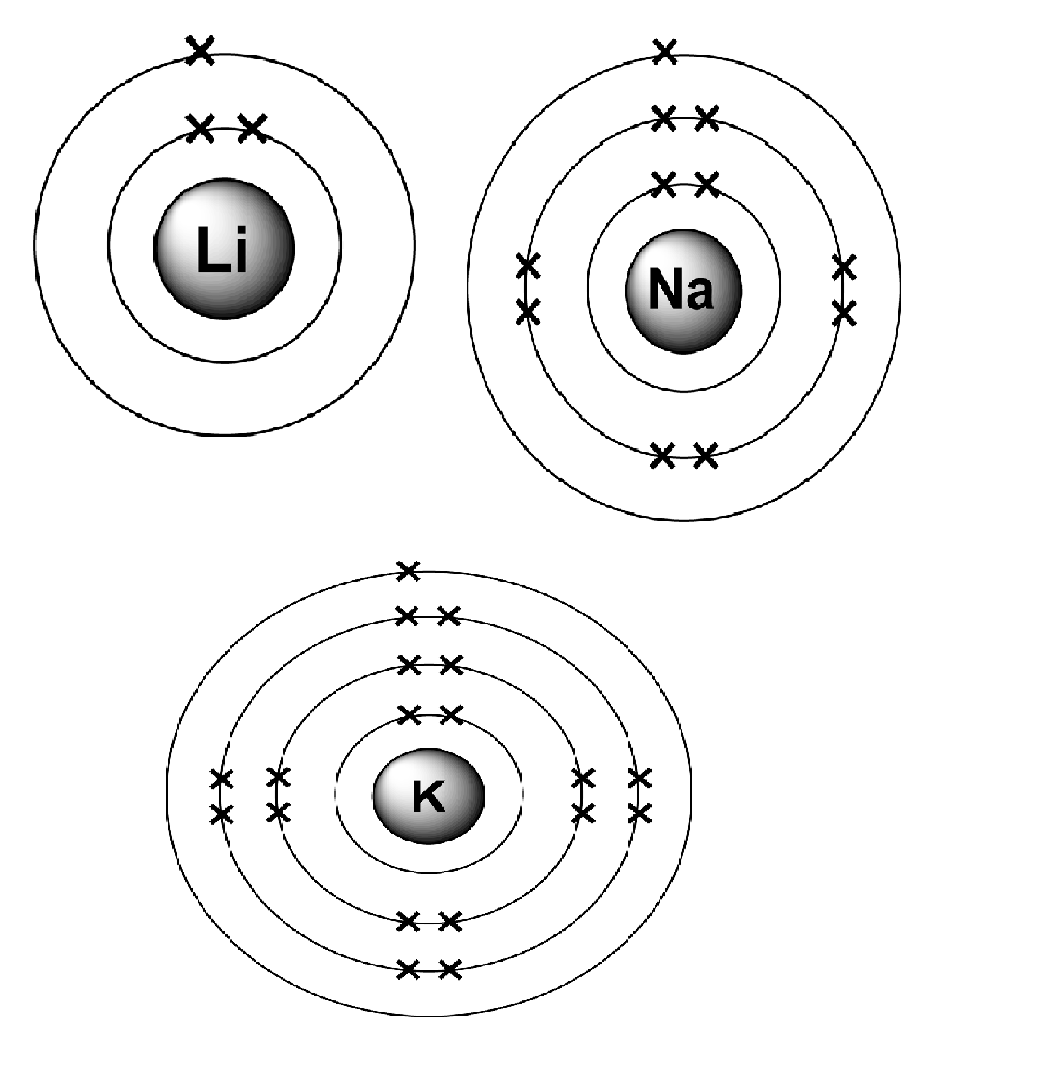
As you can see from these electronic structures, each element has the same number of outer shell electrons (1 in this case), but each time down the group, additional energy levels are occupied, lithium with 2 occupied energy levels, sodium 3, and potassium 4.
