Question #88796
1 Answer
Hydrophobic Amino Acid residues...
Explanation:
Proteins are made by concatenating Amino Acids.
All proteins in this world are made up of only 20: the proteinogenic AA's.
These all have an identical backbone (
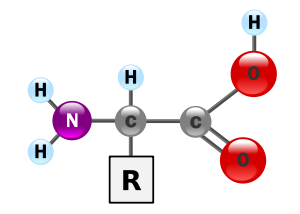
(Source: https://en.wikipedia.org/wiki/Amino_acid)
These side-chains (residues) can be hydrophilic (polar, or "water-loving") or hydrophobic (apolar, or "water-shunning").
A protein essentially is like a string of pearls, but not in a linear fashion: the identity of the residues (the R-chains) determines how the chain is formed 3D-wise.
There are three main structures:
Depending on its function an enzyme will contain a combination of these structures: (green = enzyme)
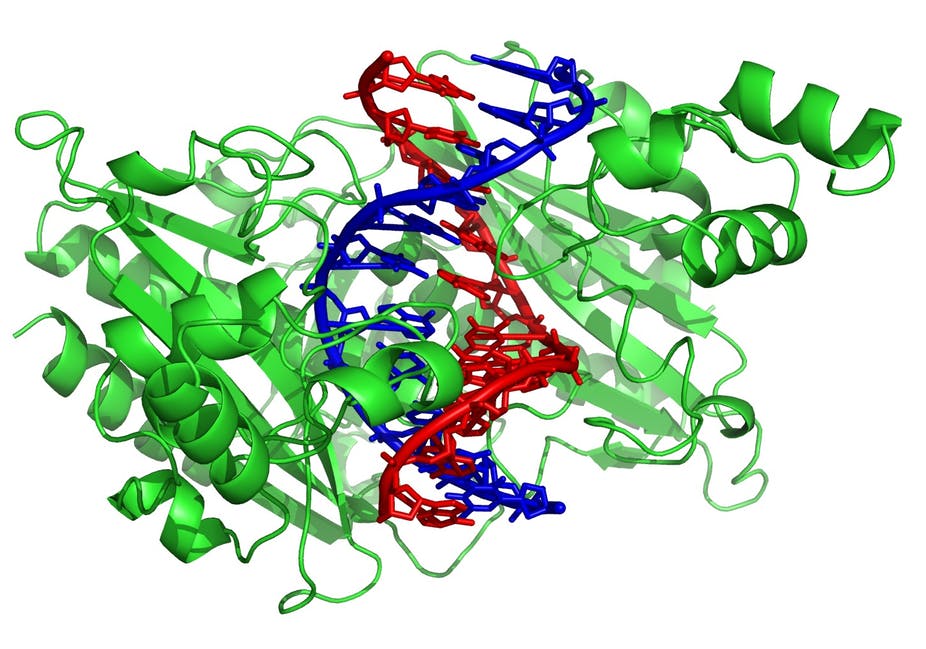
If a region is Hydrophobic, it won't be happy and seek refuge in an apolar environment, like the lipid bilayer of cell membranes.:
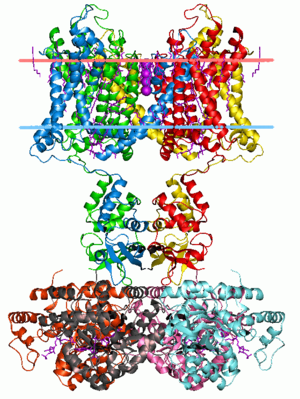
image source: https://en.wikipedia.org/wiki/Membrane_protein
In the image above, the space between the pink and the light-blue horizontal lines represents the lipid bylayer, and you can see that various
This anchors the protein (in this case a Potassium Channel) firmly in the bilayer, as the hydrophobic helices will actively resist being pulled into the polar region outside...

