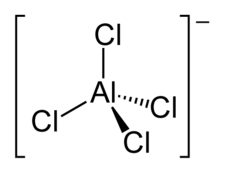
Question #66d23
2 Answers
That compound is actually an ionic compound, not an ion in and of itself.
Explanation:
Geometry Determination:
=> Bonded
=> NonBonded
=> Total bonded
=>
=> Tetrahedral Geometry
Formal and Net Charge:
Formal Charge of Element in Compound (FC) ...
FC = Valence electrons - (Bonded electrons/2) - Nonbonded electrons)
FC =
Formal Charge per element:
FC(Cl) =
FC(Al) =
Net Molecular Charge: