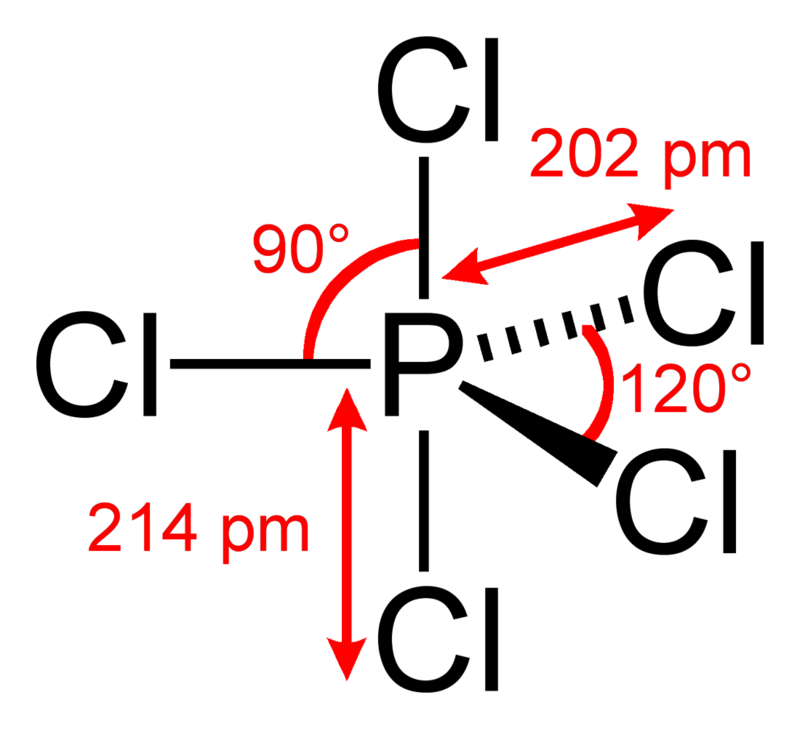
What is the structure of #PCl_5#?
1 Answer
Nov 19, 2017
Well we got
Explanation:
...the which are arranged as a trigonal bipyramid around the central phosphorus atom.
And thus
And
The equivalent structure,