Question #d0a6c
1 Answer
Explanation:
For starters, take a look in the Periodic Table and find the atomic number of sulfur,
Z_ "sulfur" = 16
Since your unknown atom contains
Z = 16 + 1 = 17
This element is chlorine,
Now, the number of neutrons present in the sulfur-36 isotope is given by the mass number of the isotope,
color(blue)(ul(color(black)("no. of neutrons" = "mass number " - " atomic number")))
For the sulfur-36 isotope, you have
{(A = 36), (Z = 16) :}
This means that this isotope contains
"no. of neutrons" = A - Z
"no. of neutrons" = 36 - 16 = 20
You can thus say that the chlorine isotope, which contains the same number of neutrons as the sulfur-36 isotope, will have a mass number equal to
A = Z + "no. of neutrons"
A = 17 + 20 = 37
This means that the unknown isotope is chlorine-37, one of two stable isotopes of chlorine. In isotope notation, which uses the atomic number, the mass number, and the chemical symbol of the element
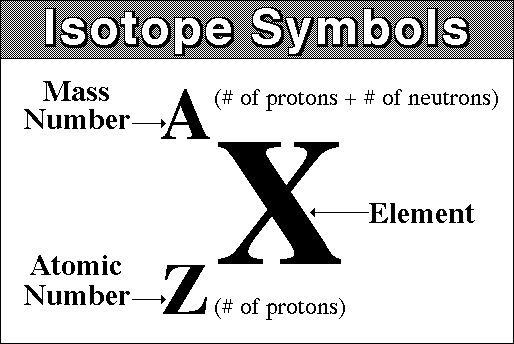
you can represent chlorine-37 as
""_17^37"Cl" -> {("mass number" = 37), ("atomic number" = 17) :}

