Question #03297
1 Answer
Explanation:
Your tool of choice here will be the Rydberg equation, which allows you to calculate the wavelength of the photon emitted when an electron in a hydrogen atom makes an
1/(lamda) = R * (1/n_f^2 - 1/n_i^2)
Here
R is the Rydberg constant, equal to1.097 * 10^(7) "m"^(-1) n_i is the energy level from which the electron fallsn_f is the energy level to which the electron falls
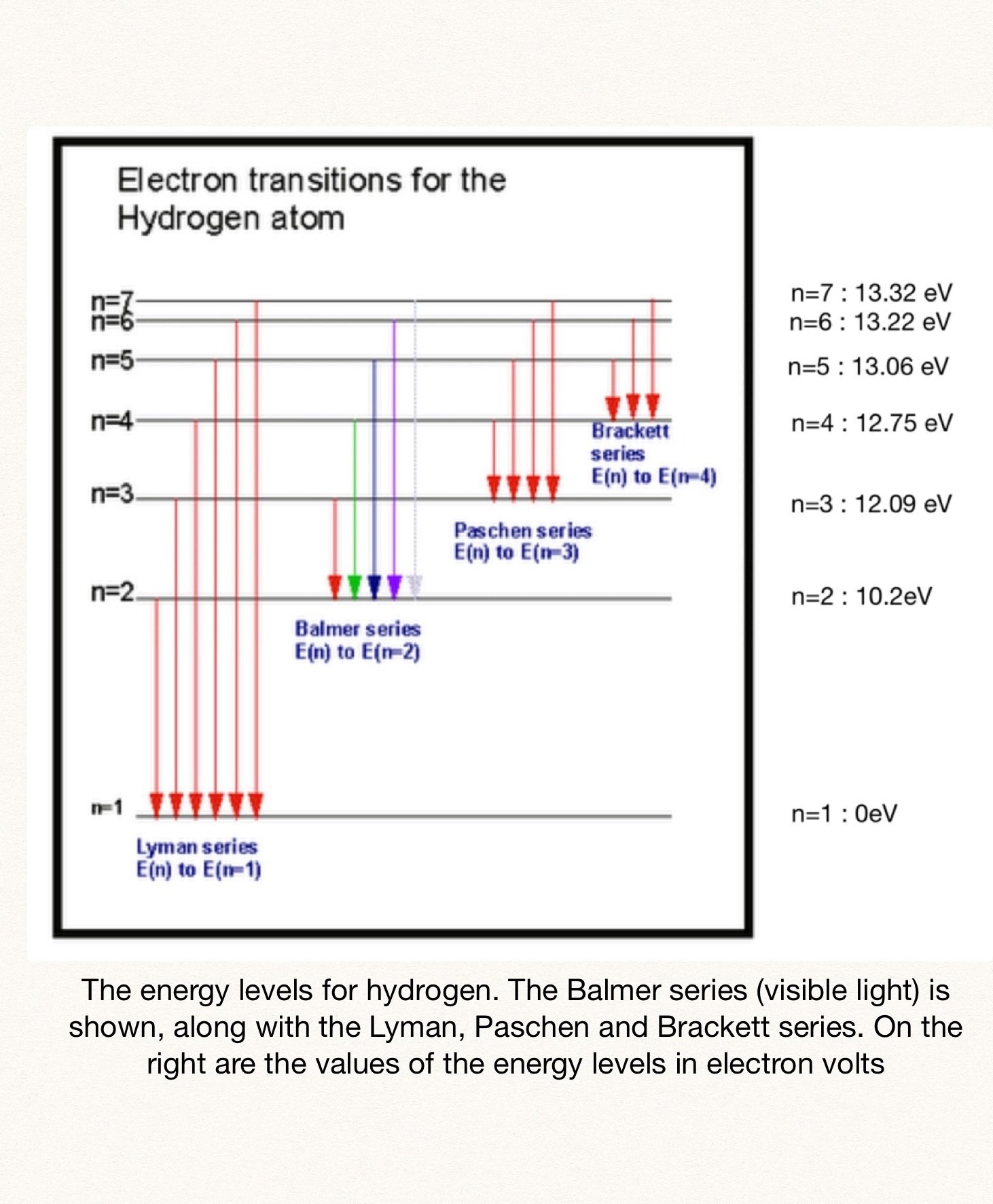
In your case, you know that the electron falls from the seventh energy level, so
n_i = 7
Your goal here is to find the value of the final energy level,
1/lamda = R * (n_i^2 - n_f^2)/(n_i^2 * n_f^2)
n_i^2 * n_f^2 = lamda * R * (n_i^2 - n_f^2)
n_i^2 * n_f^2 + lamda * R * n_f^2 = lamda * R * n_i^2
This is equivalent to
n_f^2 * (n_i^2 + lamda * R) = lamda * R * n_i^2
which gets you
n_f = sqrt( (n_i^2 * lamda * R)/(n_i^2 + lamda * R))
Now all you have to do is to plug in the values that you have--do not forget that the wavelength of the photon must be expressed in meters!
n_f = sqrt( (7^2 * 1005 * 10^(-9)color(red)(cancel(color(black)("m"))) * 1.097 * 10^7 color(red)(cancel(color(black)("m"^(-1)))))/(7^2 + 1005 * 10^(-9)color(red)(cancel(color(black)("m"))) * 10.97 * 10^7 color(red)(cancel(color(black)("m"^(-1))))))
n_f = 2.99983 ~~ 3
Therefore, you can say that a photon of wavelength
 https://thecuriousastronomer.wordpress.com/2013/08/20/emission-line-spectra/
https://thecuriousastronomer.wordpress.com/2013/08/20/emission-line-spectra/

