Are electrons involved in ionic bonding?
1 Answer
Nov 24, 2017
Electrons are involved in ionic bonding.
Explanation:
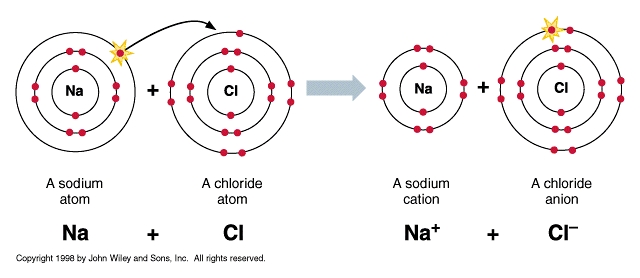
In ionic bonding, valence electrons from one atom are transferred to another atom.
Electrons are negatively charged, thus the atom that loses electrons becomes a cation (positively charged ion), and the atom that gains the electrons becomes an anion (negatively charged ion).
The positive and negative charges attract, and an ionic bond is formed between the two ions.
 http://www.physicsandmathstutor.com/chemistry-revision/ionic-bonding/
http://www.physicsandmathstutor.com/chemistry-revision/ionic-bonding/

