Question #655ff
1 Answer
It may be an
Explanation:
Let's write the reaction as
Here are the steps I suggest.
Step 1. Nucleophilic attack of the alcohol on the carbonyl group
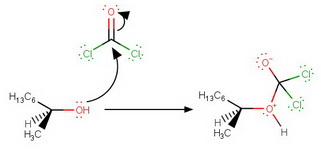
Step 2. Expulsion of
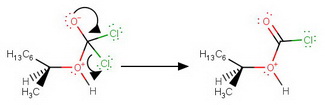
Step 3. Deprotonation by the expelled
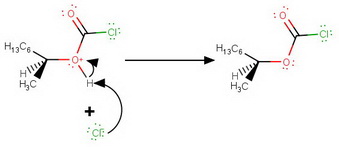
Step 4. Departure of the leaving group
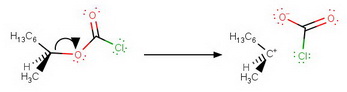
The carbocation and the chlorocarbonate ion form an intimate ion pair that is held together tightly in space.
Step 5. Attack of
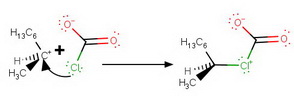
The
Step 6. Loss of
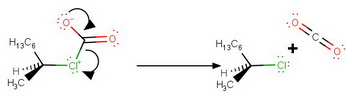
This step may be simultaneous with Step 5.

