Question #1aba7
1 Answer
Two atoms are
Explanation:
Start by drawing the Lewis structure
We have 5×7 +1 = 36 electrons to place around the five atoms.
We will minimize formal charges by arranging the electrons symmetrically.
Here's what I got.

I put 2 lone pairs on the central atom and 3 lone pairs on each of the other four.
This gives the central atom a formal charge of +1; atoms 2 and 4 get a formal charge of -1; and the terminal atoms have zero formal charge.
Hybridization of central atom
The central atom has 2 bonding pairs and 2 lone pairs (
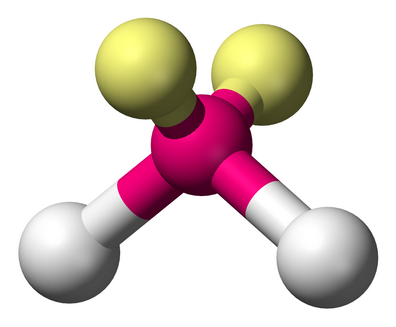
(From Wikimedia Commons)
This corresponds to a tetrahedral electron geometry and a bent molecular geometry (the yellow balls represent the lone pairs).
This corresponds to
Hybridization of atoms 2 and 4
Atoms 2 and 4 each have 2 bond pairs and 3 lone pairs (

(Adapted from Wikimedia Commons)
This corresponds to a trigonal bipyramidal electron geometry and a linear molecular geometry.
This corresponds to an
Thus, two atoms are

