Question #291bf
1 Answer
Dec 4, 2017
To provide the activation energy necessary to start the reaction.
Explanation:
The activation energy of a reaction is the energy necessary in order to break all of the reactants' bonds, so they can reform new bonds to make products. The activation energy can be fulfilled by applying heat energy.
Below is a diagram of the activation energy for an exothermic reaction. An exothermic reaction is a reaction where the energy of the products is lower than the energy of the reactants. The excess energy is released as heat energy.
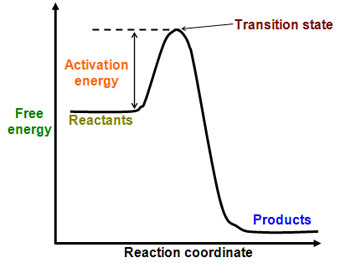
Here is an energy diagram for an endothermic reaction, where the products have more energy than the reactants:
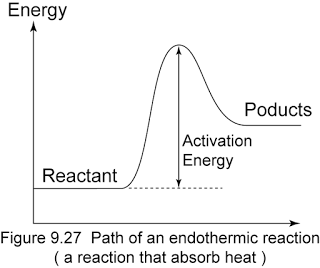
Hope this helps!

