Question #92f86
1 Answer
Dec 4, 2017
Explanation:
The atoms within the layer are more strongly bonded to atoms in adjacent layers. This gives
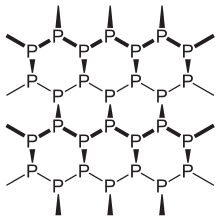
The atoms within the layer are more strongly bonded to atoms in adjacent layers. This gives
