What is the atomicity of sodium nitrate?
1 Answer
Dec 8, 2017
Excuse me while I check what
Explanation:
You gots
A resonance structure of the nitrate ion features charge separation in that the nitrogen atom is QUATERNIZED, and bears a formal positive charge. And given this condition, TWO of the oxygen atoms must bear a formal negative charge in order to give an overall negative charge on the molecule....
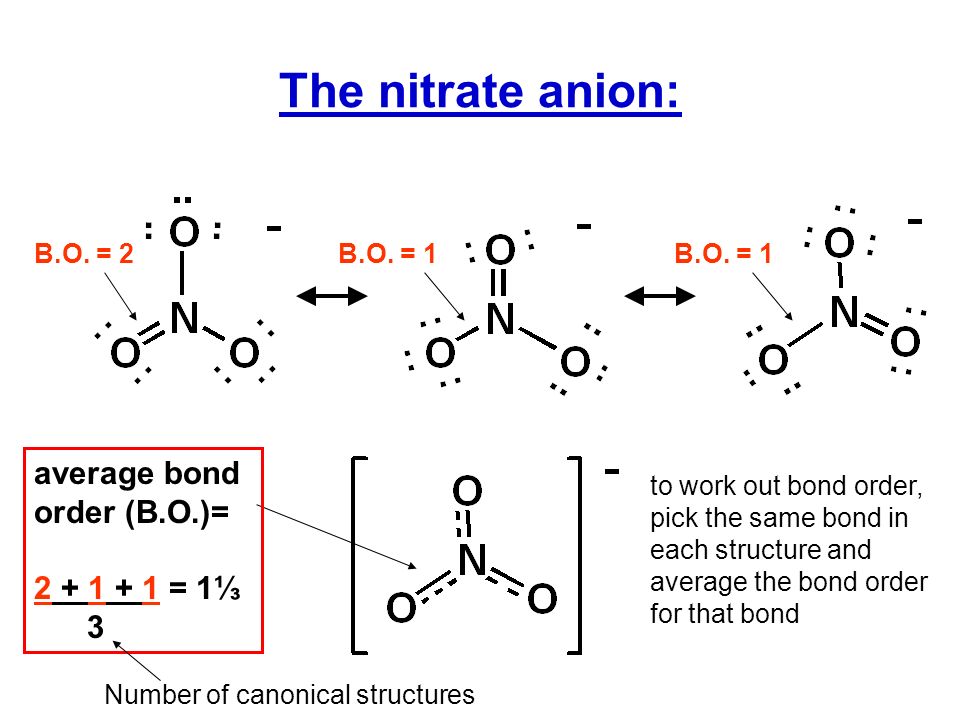
Of course I can distribute the negative charges about the oxygen centres....

