Question #d6832
1 Answer
They refer to the symmetry of the orbitals in a molecule.
Explanation:
You may have seen molecular orbital diagrams like the one below for

The terms refer to orbitals that have a centre of symmetry(also called a centre of inversion).
Inversion consists of drawing a line from some point (x, y, z) in an orbital through its mid-point and out the same distance to a point (-x, -y. -z) on the other side of the orbital.
If inversion results in the same phases for the molecular orbital, then the MO has gerade (g) symmetry.
If inversion results in opposite phases for the molecular orbital, the MO is has ungerade (g) symmetry.
The German words "gerade" and "ungerade" mean "even" and "odd", respectively.
Let's apply this concept to
(a)
If two
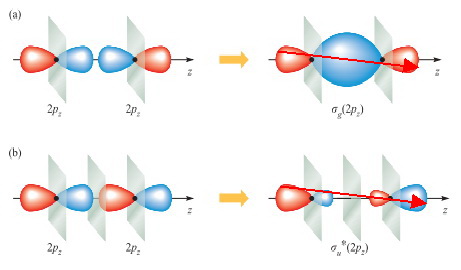
(Adapted from Physics Stack Exchange)
If we draw a line from the upper left, through the mid-point, to the lower left of the orbital, the phase of the orbital remains unchanged ( red → red).
Thus,this orbital is gerade (g).
(b)
If two
If we draw a line from the upper left, through the mid-point, to the lower left of the orbital, the phase of the orbital changes ( red → blue).
Thus, this orbital is ungerade (u).
Here's how you pronounce the word "ungerade":

