Question #bcd01
1 Answer
Dec 19, 2017
One orbital per C-H bond
Explanation:
The p-orbital involved in the C-H bond is co-planar with the empty p-orbital in the methyl carbocation. This geometry means that hyperconjugation can occur, with the electrons in the
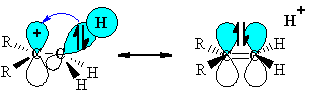 http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch04/ch4-3-2-1.html
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch04/ch4-3-2-1.html
The stabilisation is energetically favourable, because it results in the electrons in the
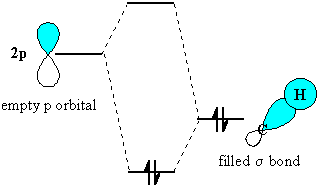 http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch04/ch4-3-2-1.html
http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch04/ch4-3-2-1.html
The C-C bond can rotate, so the carbocation can form stabilising interactions with multiple C-H bonds e.g. ethyl cation has 3 C-H bonds that can be involved in hyperconjugation.

