If the chemical formula is #"C"_6"H"_10"O"_3#, what is the degree of unsaturation? Then, given the information below, what is the compound?
The #""^(1) "H"# #"NMR"# has a peak at 2.5 ppm with 6 protons and 2 neighbors and a peak near 1.2 ppm with 4 protons and 3 neighbors.
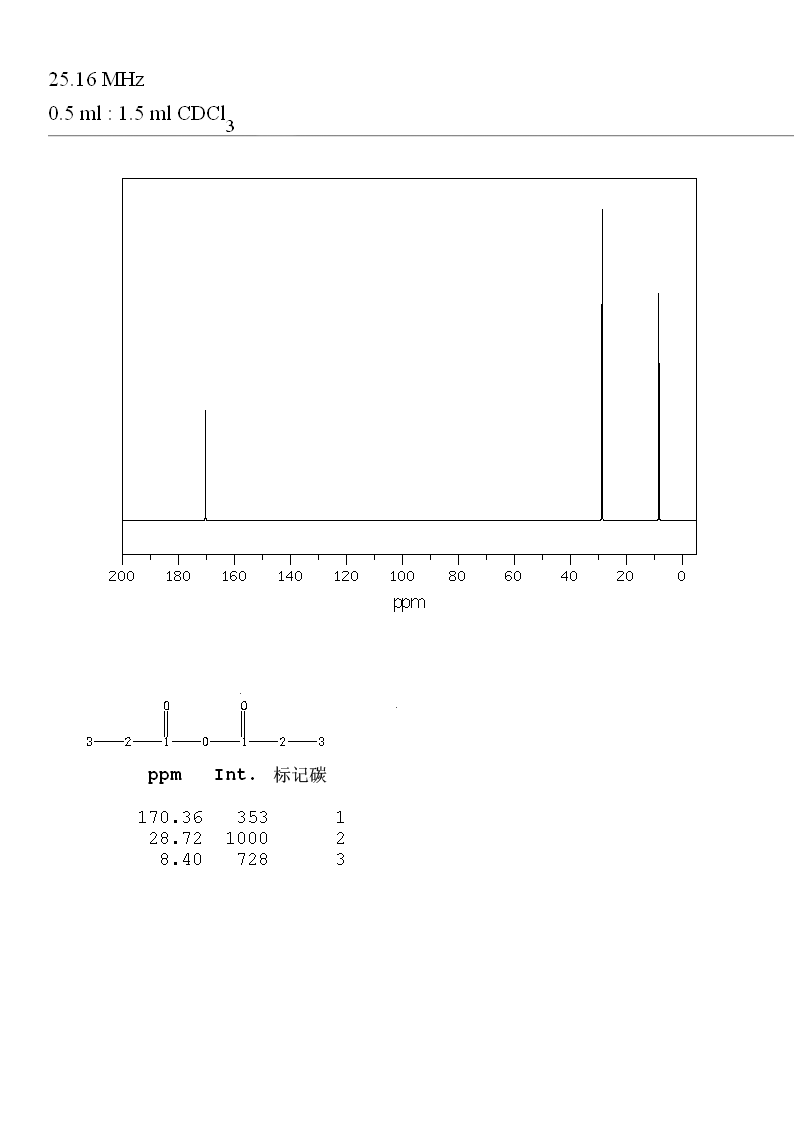
The #""^(13) "C"# #"NMR"# has singlet peaks at 8 ppm, 28 ppm, and 170 ppm.
The
The
2 Answers
Your question might be "1.2 ppm with 6 protons and 2.5 ppm with 4 protons."
Explanation:
The spectra has only two peaks for
I surfed the Internet and found the "answer": Propionic anhydride.
However, the structure of propionic anhydride is inconsistent with "2.5 ppm with 6 protons and 2 neighbors and a peak near 1.2 ppm with 4 protons and 3 neighbors".
If the conditions are "
Warning! Long Answer. The compound is propionic anhydride.
Explanation:
Preliminary analysis
You know the formula is
An alkane with six carbon atoms has the formula
The degree of unsaturation
Therefore, the compound contains two rings and/or double bonds.
The spectrum has 10 protons and only two peaks. The molecule must have a symmetrical structure.
A peak with 2 neighbours and aone with 3 neighbors corresponds to an ethyl group (
The
However, I think you have the assignments reversed. The methyl group should have the smaller chemical shift.

A methyl group is normally at 0.9 ppm. Something is pulling it downfield
to 1.2 ppm.
A methylene group is normally at 1.3 ppm. Something is pulling it downfield
to 2.5 ppm.
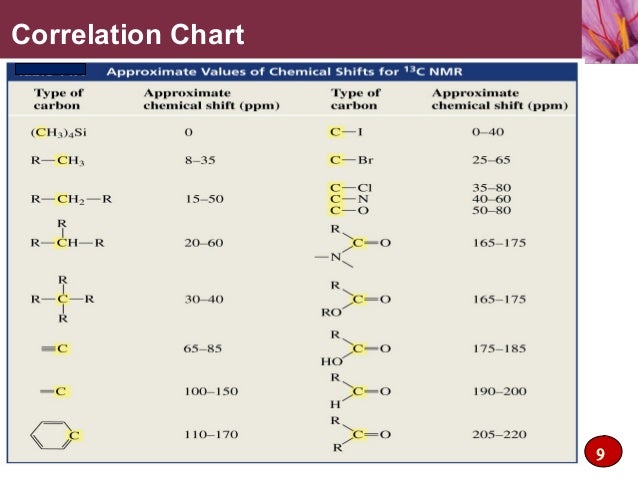
We see from the table that a
We could expect a similar 1.3 ppm shift for a
This is just where the
We now know that the partial structure is
These fragments add up to
There is only one
The compound is propionic anhydride.
Confirmation:
1. The compound has two double bonds.
2. Three
We should expect to see
#"CH"_3color(white)(mm)# at 10 ppm#"CH"_2 color(white)(mm)# at 30 ppm#"(C=O)O"# at 170 ppm
We see peaks at 8 ppm, 28 ppm, and 170 ppm. This is consistent with propionic anhydride.
3. The