Question #e7673
1 Answer
Jan 6, 2018
Phenolphthalein is a suitable indicator because it changes colour at the equivalence point of the titration.
Explanation:
Here's a typical titration curve for acetic acid.
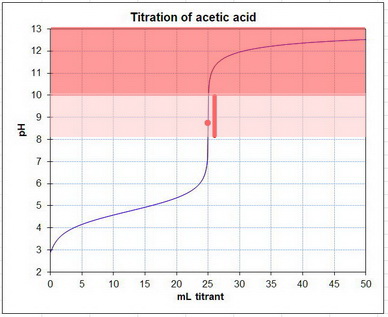
The equivalence point is near pH 9 (the red dot).
Phenolphthalein changes colour from colourless at pH 8.2 to red at pH 10.
Once you have reached the pale pink endpoint, adding one more drop of the base will turn the indicator to red.

