Question #be4c4
1 Answer
Warning! Long Answer. Usually, we titrate an amino acid to get information about its acid-base properties.
Explanation:
The formula of alanine is often written as
Alanine has two dissociation steps.
The "neutral" form, which has one positive and one negative charge, is called a zwitterion (double ion).
Fron the titration curve, we can deduce the values of
#"p"K_text(a₁)# #"p"K_text(a₂)# #"pI"# , the isoelectric point, i.e. the pH at which the alanine exists mainly as a zwitterion
Here is a schematic titration curve for alanine.
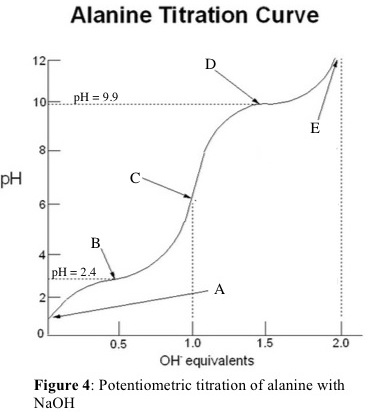
(Adapted from Chegg)
Some points to note from the curve are:
(a) The graph has two "waves", showing that we have a diprotic acid.
(b) The two equivalence points occur after adding 1 eq of base (Point C) and 2 eq of base (Point E).
(c) The alanine is fully protonated at pH 1 (Point A), where it exists as a cation.
(d) After adding 0.5 eq base (Point B), we have deprotonated half of the carboxyl groups. At this point,
(e) After adding 1 eq base (Point C), we have reached the first equivalence point and deprotonated all of the carboxyl groups. The alanine now exists primarily as a zwitterion, with no net charge.
(f) After adding 1.5 eq base (Point D), we have deprotonated half of the ammonium groups. At this point,
(g) After adding 2 eq base (Point E), we have reached the second equivalence point and deprotonated all of the carboxyl groups. The alanine now exists as an anion.
(h) Point C is the isoelectric point. Even though it may be difficult to measure the pH from the graph, we can calculate it from the relation

