Question #bc581
1 Answer
(a) Nothing; (b) nothing; (c) a colour change; (d) evolution of a gas.
Explanation:
(a) Acetic acid + phenolphthalein
You will observe nothing.
Water is slightly acidic because it absorbs carbon dioxide and forms carbonic acid.
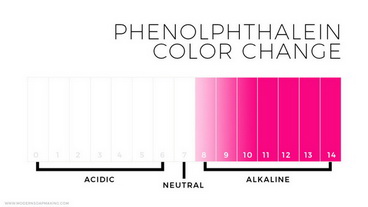
Phenolphthalein is an acid-base indicator. It is colourless in acidic solution.
(From Modern Soapmaking)
You are going from a slightly acidic to a more acidic solution, so you will observe no change.
(b) Acetic acid + distilled water
Both solutions are colourless. You will observe no change.
(c) Acetic acid + universal indicator
If your initial solution is neutral, the colour will be green.

When you add the acetic acid, the colour will change to yellow or beige.
(d) Acetic acid + sodium hydrogen carbonate
You will observe the evolution of a colourless gas.
Sodium hydrogen carbonate in the presence of acid decomposes to form carbon dioxide.

