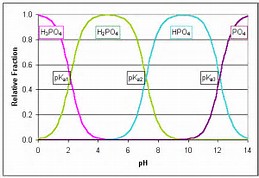
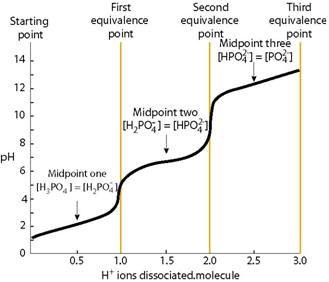
Why is the third ionization of phosphoric acid, #H_3PO_4#, disfavoured?
1 Answer
Jan 22, 2018
Well, simply because phosphoric acid IS NOT a triacid.
Explanation:
At most it is a diacid in aqueous solution; and titration with sodium hydroxide would give an endpoint as
This site reports the acidities of phosphoric acid...
The third dissociation DOES not occur to any extent in aqueous solution, as the second graph shows.....