What is the Lewis structure of #XeF_2O#? What should be its geometry?
1 Answer
Well we got
Explanation:
And so we got 14 electron pairs....and this is easier to represent on paper than it is on this editor.
We gots 3 bonding electron pairs, and two lone pairs around the central xenon atom. Electronic geometry is
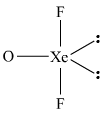
And for a Lewis structure of
Given the influence of the lone pairs....we would anticipate that

