Question #2943c
1 Answer
Jan 25, 2018
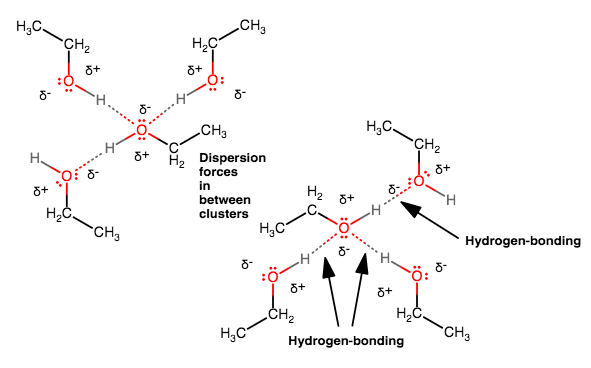
Ethanol can hydrogen-bond and has some small dispersion forces. Everything has dispersion...
The electronegative
- The lone pairs on each
#"O"# can act as hydrogen-bond acceptors. - The
#bb"H"# bonded to the#"O"# can act as a hydrogen-bond donor.
Clusters of ethanol molecules can then interact via weak dispersion forces via their hydrocarbon tails

