What is allosteric inhibitor?
1 Answer
A non-competitive inhibitor which attaches to the enzyme at allosteric site i.e any place on enzyme except active site, is called allosteric inhibitor.
Explanation:
An allosteric inhibitor by binding to allosteric site alters the protein conformation in active site of enzyme which consequently changes the shape of active site. Thus enzyme no longer remains able to bind to its specific substrate. Hence enzyme is unable to perform it's catalytic activity i.e enzyme is now inactive. This process is called allosteric inhibition.
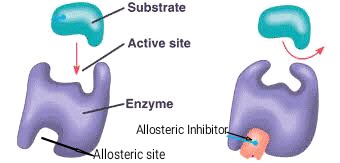
For example:
Hope it helps...

